Abstract
Diffuse Large B Cell Lymphoma (DLBCL) is the most common form of B-cell Non-Hodgkin Lymphoma (NHL), representing a third of all new cases. DLBCL is further sub-divided into various molecular sub-types based on gene expression and co-occurring genetic alterations. Gene expression based- subtypes include the germinal center B-cell (GCB) and the activated B-cell type (ABC) subtypes, with the ABC sub-type having a poorer prognosis than the GCB sub-type. Interestingly, 30-40% of all DLBCL patients harbor mutations in key epigenetic regulators, EZH2, KMT2D, CREBBP, EP300, and mutations in histone proteins themselves.
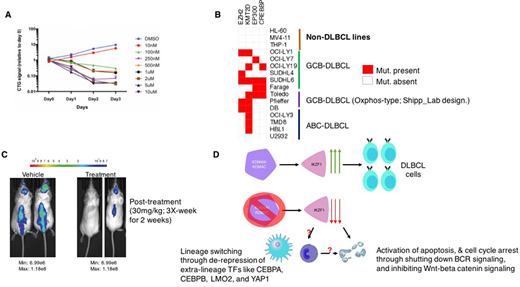
Through an unbiased cell-based phenotypic screen, we discovered that inhibition of lysine demethylases, specifically KDM4C and KDM4A, represents a vulnerability across 15 different DLBCL cell lines including germinal center B-cell (GCB) and activated B-cell (ABC) type lines, with a GI50 value between 75nM to ~200nM, while sparing leukemia lines. Consistently, treatment of xenograft-based animal models of DLBCL with a low dose of KDM4A/KDM4C inhibitor delivered intra-peritoneally three times a week, results in a drastic reduction of tumor burden. Both KDM4A and KDM4C catalyze the removal of histone H3 K9 di- and tri-methylation (H3K9me2/3) and H3K36 di- and tri-methylation (H3K36me2/3). H3K9me2/3 is associated with promoter and enhancer repression, while H3K36me2/3 is present in gene bodies during transcription but also functions as a chromatin repressor Consistently, we have identified key enhancers, including those associated with IKZF1, that are "decommissioned" after inhibition of KDM4A/KDM4C. Repressed enhancer activity, through loss of H3K27 acetylation and H3K4me1, and gain of H3K9me2/3, results in a rapid transcriptional downregulation of IKZF1 and its partners including IKZF3. Given the role of IKZF1 in the transcriptional regulation of key B Cell Receptor (BCR) signaling components, we show that KDM4A/KDM4C inhibition leads to a downregulation of SYK, a proximal BCR-signaling component, which likely precedes DLBCL cell apoptosis. In addition, we observed an activation of extra-lineage transcription factors such as CEBPA and CEBPB, which are normally repressed by IKZF1 in the lymphoid lineage. A concomitant downregulation of the B-cell gene expression program and an upregulation of the myeloid (CD14+ monocytic) gene expression program is also observed, implying a "trans-differentiation" of DLBCL cells into the monocyte lineage. This lineage-switch correlates with an increased population of CD14+ expressing cells. Finally, using DLBCL patient data sets, we can show that over-expression of either KDM4A or KDM4C is associated with poor prognosis in DLBCL patients. In summary, we have discovered that KDM4A/KDM4C inhibition results in an increase of repressive histone modifications at several intra-genic "enhancers" of genes that are responsible for the survival and proliferation of DLBCL cells. The elucidation of this unique epigenetic mechanism provides a strong rationale for the development of novel targeted therapies against both multiple subtypes of DLBCL.
Shipp:Bayer: Research Funding; AstraZeneca: Honoraria; Merck: Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal